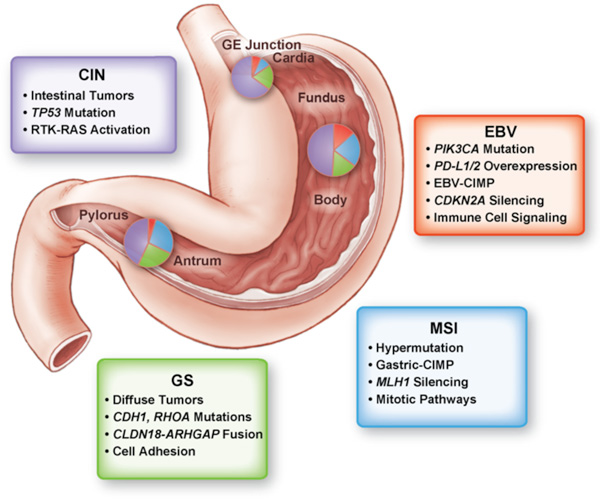
Cancer is a disease of the genome. Over time, the accumulation of mutations either activating genes that promote cellular growth or inactivating those genes that suppress growth ultimately can lead to cancer. Identifying the essential genes that underlie cancer provides an entry point to understand the biology of cancer and, critically, develop new therapies. The recent explosion of genomic technologies has allowed us to study cancer in ways that would have seen unimaginable just a few years ago. With these great opportunities also come great challenges. Our new view into the genome is not a simple roadmap toward improving therapy. Rather, we now are faced with a large puzzle to piece together.
In our laboratory, we bring together expertise in modern genomics, experimental/functional biology and clinical medicine. Our overarching goal is to leverage the study the cancer genome to elucidate key biological processes and therapeutic vulnerabilities in carcinomas arising in the GI tract, with a greatest focus on stomach and esophageal cancers. Our work has traditionally started from an understanding of the genomic alterations underlying these cancers. Working closely with the Broad Institute through our leadership in The Cancer Genome Atlas projects, we have been at the forefront of several major initiatives to interrogate the genomes of these cancers. We have identified critical oncogenes and tumor suppressors and developed new classification systems for categorizing these cancers. Moving forward we are addressing a number of key questions.
- Continued Genomic Exploration: While we have learned incredible amounts from the cancer genome, there are massive questions remaining. Emerging question include how aneuploidy mediates tumorigenesis, defining tumor heterogeneity, defining the of plasma DNA, and linking genomic profiling to patient outcomes.
- Mechanistic Understanding of Gastroesophageal Oncogenes: There are a host of key cancer promoting genes for which their precise oncogenic function is not known. We are now performing detailed functional assessment of select cancer promoting genes to define their actions. These studies span the use of traditional cell line models and also novel engineered mouse and organoid systems.
- Defining Therapeutic Strategies: Genomic profiling has already identified bona fide targets in these cancers such as amplified receptor tyrosine kinases such as ERBB2/HER2. However, finding a target is not the same as defining a therapeutic strategy. We are working to define optimal combination therapy for key targets, to define mechanisms of resistance and biomarkers for new therapies. These studies span analysis of patient samples, use of traditional cell line models as well as novel patient-derived cancer models and new engineered mouse systems.
- Identifying Novel Vulnerabilities: Working closely with the Broad Institute, we are leveraging new technologies and approaches for functional genomic screening and chemical screening to ask specific questions regarding dependencies in these cancers. These studies are both a foundation to define new areas of biologic interest and to define new candidate targets.
- The Tumor Microenvironment and Cancer Immunology: A tumor is more than cancer cells. The recent explosion of immune therapies is rapidly changing oncology and cancer research. Expanding work in our group is evaluating the tumor microenvironment of gastric and esophageal cancers, exploring the intersection of the somatic genomic aberrations and immune system and exploring the optimal targets for immunotherapy. These studies span both evaluation of human tissues and experimental studies with murine models.
We are entering an era where genomic and molecular profiling of each patient’s tumor will be an integral factor in guiding cancer care. In sum, our laboratory is committed helping to develop a biologic foundation that will enable clinicians and researchers to be able to utilize these molecular data in real clinical time to positively impact the care of our patients.
 |
 |
 |
 |
